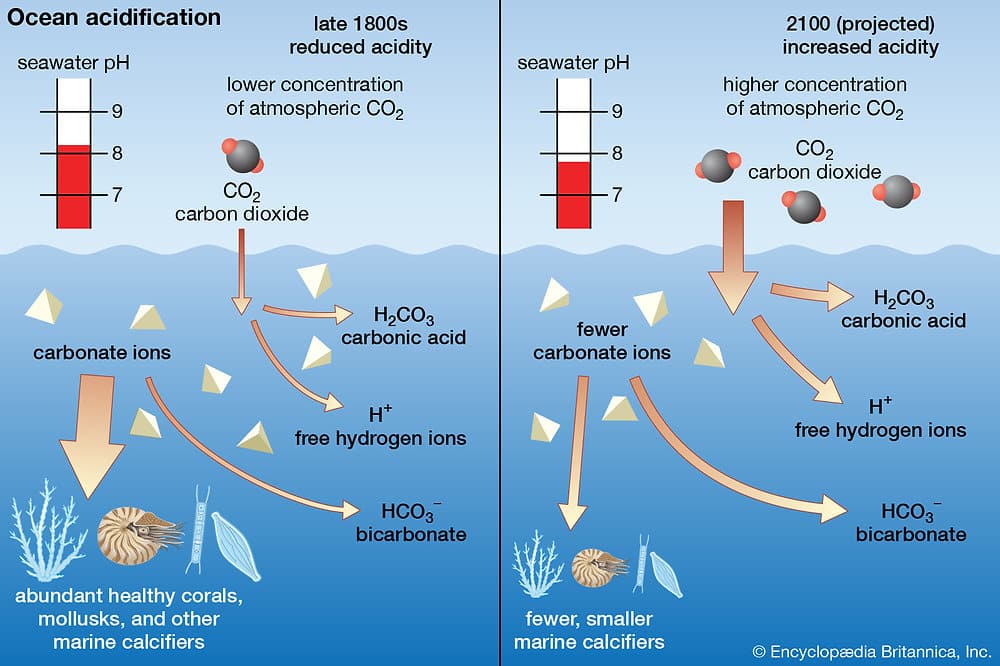
- The ocean, which absorbs about 30% of the carbon dioxide (CO2) produced by humanity, is undergoing an alarming transformation: its acidification. This phenomenon, marking a decrease in the pH of seawater, occurs over a period spanning decades. According to the IPCC, this drop in pH mainly results from human activities, exacerbating the stresses that oceans are already facing.
- These chemical transformations not only disrupt the natural balance, but also worsen the impact of climate change on our planet. The proliferation of acidity affects not only the oceans but also disrupts terrestrial ecosystems, particularly by altering the average state of aragonite saturation, which is essential for many marine organisms.
- The complex interaction between ocean acidification and global warming illustrates the need for a thorough understanding of these phenomena in order to propose viable solutions.

Ocean acidification is one of the most concerning phenomena of our time, primarily caused by the increase in atmospheric carbon dioxide (CO2). It alters the chemistry of the ocean, influencing marine ecosystems, coastal economies, and the global climate. This article explores how ocean acidification is redefining our planet in several key aspects.
Table of Contents
ToggleThe chemical dynamics of ocean acidification
The oceans absorb about 30% of the carbon dioxide (CO2) emitted by human activities. This process, while beneficial for reducing atmospheric CO2, leads to a chemical reaction with seawater, decreasing the ocean’s pH and increasing its acidity. Historically, the average pH of surface water was 8.2 but has noticeably dropped due to human activity.
Impacts on marine ecosystems
Ocean acidification has a profound impact on marine life. For example, it affects calcifying organisms such as corals, mollusks, and certain crustaceans, whose shells and skeletal structures are primarily composed of aragonite. The reduction in aragonite saturation makes the construction and maintenance of these structures increasingly difficult, thereby threatening their survival and the balance of the ecosystems that depend on them.
Economic and social consequences
Ocean acidification poses a threat to the global economy, particularly for coastal communities dependent on fishing and marine tourism. The degradation of coral reefs, for instance, reduces biodiversity, impacts fishing, and diminishes the attractiveness of tourist destinations. As a result, the livelihoods of millions of people are at risk.
Ocean acidification and climate change
The link between ocean acidification and climate change is complex. Oceans play a fundamental role in regulating the climate by absorbing CO2 and heat. However, increased acidification could diminish the oceans’ capacity to store CO2, thereby exacerbating climate change. In turn, the effects of climate change, such as ocean warming, can intensify acidification.
Solutions and future prospects
Mitigating the impact of ocean acidification requires a comprehensive and coordinated approach. Reducing CO2 emissions is crucial to slowing this trend. Efforts for ecological restoration, such as coral reef regeneration and marine habitat preservation, must be intensified. Furthermore, strengthening scientific research and international cooperation is essential for better understanding and managing the challenges posed by ocean acidification.